The Evidence Speaks Series is a recurring feature highlighting the latest in CHÉOS research. This series features summaries of select publications and is designed to keep media and the research community up to date with CHÉOS’ current research results in the health outcomes field.
To ensure this research is quick and easy to share, we are now providing social cards that you are free to save and use as you see fit.
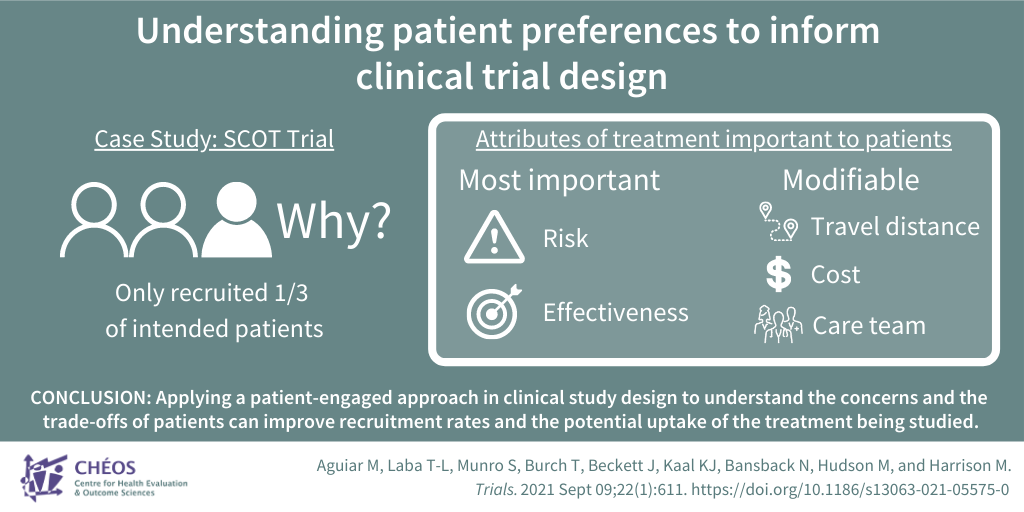
Understanding the treatment preferences of patients informs clinical trial design
Aguiar M, Laba T-L, Munro S, Burch T, Beckett J, Kaal KJ, Bansback N, Hudson M, Harrison M. Co-production of randomized clinical trials with patients: A case study in autologous hematopoietic stem cell transplant for patients with scleroderma. Trials. 2021 Sep 09;22(1):611.
Dr. Mark Harrison, CHÉOS Scientist, published a clinical trial case-study with CHÉOS Program Head of Decision Sciences Dr. Nick Bansback and Scientist Dr. Sarah Munro looking at the Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial. The SCOT trial tested a stem cell transplantation treatment for severe scleroderma, an autoimmune disorder that causes healthy skin, and sometimes internal organs, to build up excess scar tissue which is often fatal. Although the treatment showed promise, the trial had challenges recruiting patients with only 33 per cent of the trial participant target recruited. Focus groups and an online discrete choice experiment (DCE) survey were used to gather patient perspectives about scleroderma treatment. Among the seven aspects of treatment found to be important to respondents — effectiveness, immediate and long-term risk, care team composition and experience, cost, and travel distance — deterrents of participation were largely due to concerns about transportation and insurance coverage. Dr. Harrison’s study then surveyed 278 people with scleroderma and found that treatment effectiveness and risk of late complications contributed the most to participants’ decisions about whether to participate in a clinical trial, but modifiable factors of distance to treatment center and cost also impacted their decision. To illustrate, offering a treatment closer to home at lower patient cost with holistic, multidisciplinary care could increase participation by up to 51 per cent. This research emphasizes the importance of a patient-engaged approach to understand the concerns and the trade-offs people are willing to inform clinical study design, improve recruitment rates, and potential uptake of the treatment being studied by considering patient preferences.
—
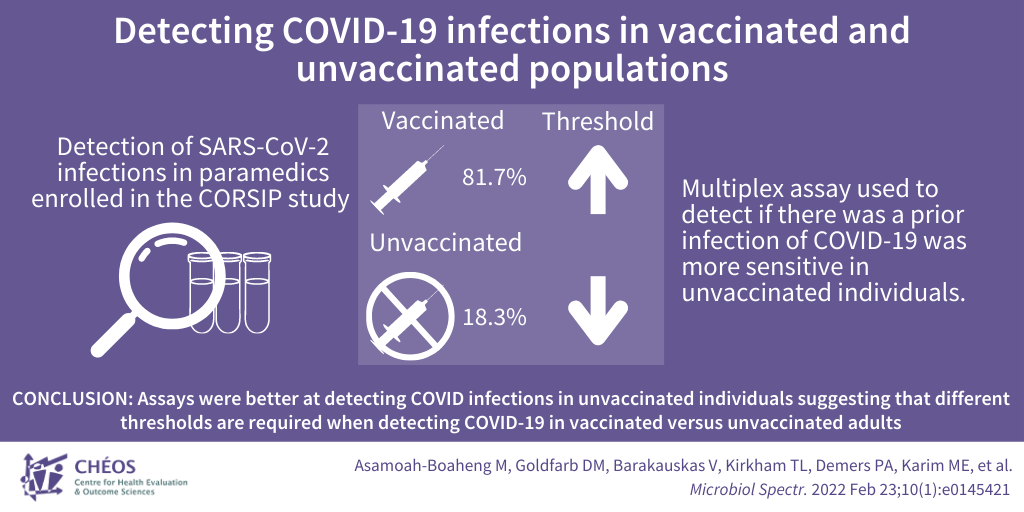
Differences in detecting prior COVID-19 infections between vaccinated and unvaccinated populations
Asahmoah-Baoheng M, Goldfarb DM, Barakuaskas V, Kirkham TL, Demers PA, Karim ME, Lavoie PM, Marquez AC, Jassem AN, Jenneson S, MacDonald C, Grunau B. Evaluation of the Performance of a Multiplexed Serological Assay in the Detection of SARS-CoV-2 Infections in a Predominantly Vaccinated Population.Microbiol Spectr. 2022 Feb 23;10(1):e0145421.
The CORSIP study, led by CHÉOS Scientist Dr. Brian Grunau, is investigating the impact of COVID-19 on paramedics. In a recent publication, supported by CHÉOS Scientist Dr. Ehsan Karim, the performance of antibody testing was evaluated to accurately detect prior SARS-CoV-2 infection and distinguish it from immune activity stimulated by COVID-19 vaccination. The population studied were paramedics enrolled in the CORSIP study, of which 81.7 per cent were vaccinated. Serum samples were tested using an assay designed to detect antibodies to antigens from SARS-CoV-2 and its known variants. Researchers found that the assay was better at detecting previous COVID-19 infections in unvaccinated individuals compared to those who had been vaccinated. The researchers concluded that different thresholds are required when detecting COVID-19 infection within the preceding 9 months in vaccinated versus unvaccinated adults.
—
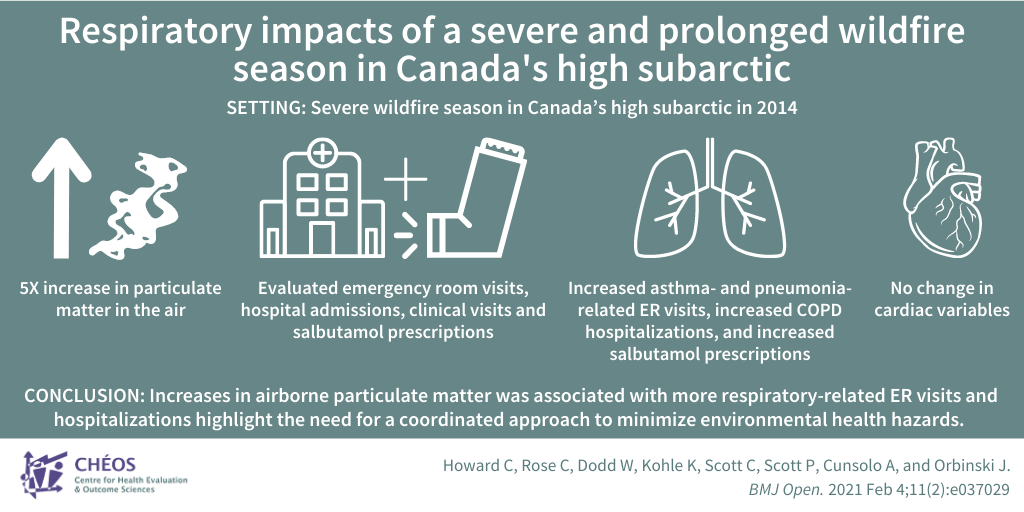
Impact of a severe wildfire season on respiratory health in the Canadian high subarctic
Howard C, Rose C, Dodd W, Kohle K, Scott C, Scott P, Cunsolo A, and Orbinski J. SOS! Summer of Smoke: A retrospective cohort study examining the cardiorespiratory impacts of a severe and prolonged wildfire season in Canada’s high subarctic.BMJ Open. 2021 Feb 4;11(2):e037029.
CHÉOS Scientist Dr. Caren Rose co-authored a retrospective cohort study looking at the cardiac and respiratory impacts of a severe wildfire season in Canada’s high subarctic. The wildfires led to a five-fold increase in particulate matter in the air during the summer of 2014, compared to 2012, 2013, and 2015. This decrease in air quality prompted public health advisories to encourage staying indoors; however, a phone survey determined that less than half of respondents spent less time outdoors and nearly one fifth reported a significant decrease in physical activity. The study evaluated emergency room visits, hospital admissions, and clinical visits for cardiorespiratory events and salbutamol (inhaler) prescriptions for patients who lived in Yellowknife and the surrounding areas. The increase in particulate matter due to the fire was associated with increased asthma- and pneumonia-related ER visits and increased COPD hospitalisations. Inhaler prescriptions also increased by 48 per cent during that summer. There were no notable changes in cardiac variables. This study demonstrates a need for active adaptation efforts to include attention to systemic factors, for example improving access to clean air shelters for recreational activities and encouraging people to go outside during times of improved air quality. The researchers called for a global approach to minimize the health impacts from environmental causes and improve coordination between public health, primary care, and government leaders to minimize environmental health hazards and address climate-related health impacts.