The Evidence Speaks Series is a recurring feature highlighting the latest in Advancing Health research. This series features summaries of select publications and is designed to keep media and the research community up to date with the Centre’s current research results in the health outcomes field.
To ensure this research is quick and easy to share, you are welcome to save the social cards and use as you see fit.
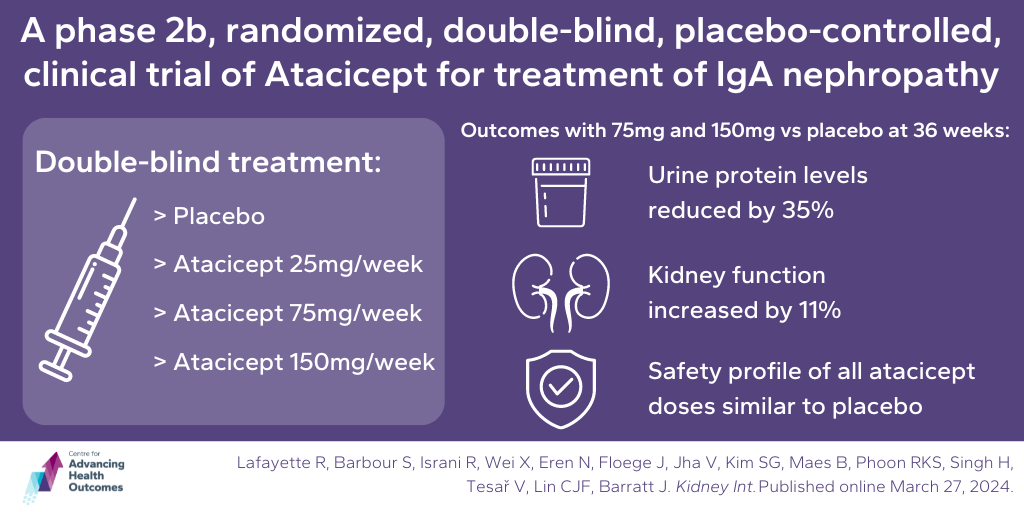
A new drug, atacicept, could help treat IgA nephropathy
Lafayette R, Barbour S, Israni R, Wei X, Eren N, Floege J, Jha V, Kim SG, Maes B, Phoon RKS, Singh H, Tesař V, Lin CJF, Barratt J. A Phase 2b, randomized, double-blind, placebo-controlled, clinical trial of atacicept for treatment of IgA nephropathy. Kidney Int. Published online March 27, 2024.
Atacicept, a new medication, was tested in a clinical trial for its effectiveness in treating IgA nephropathy, a kidney disease caused by deposits of the protein immunoglobulin A (IgA) inside the filters in the kidney. Advancing Health Scientist, Dr. Sean Barbour, worked with an international team on the study, which involved 116 participants with this condition. They were divided into four groups and given different doses of atacicept (150, 75, or 25mg) or a placebo for up to 36 weeks. The team measured the amount of protein in the participants’ urine, a sign of kidney damage. The results showed that atacicept significantly reduced protein levels in the urine compared to the placebo, indicating its potential to improve kidney health. The higher doses of atacicept were more effective and showed results sooner than the lower doses. Additionally, the medication helped stabilize kidney function, which is crucial for overall health. Importantly, the safety of atacicept was similar to the placebo, meaning it didn’t cause any unexpected side effects. These findings suggest that larger clinical trials should be done to confirm the benefits of atacicept in treating IgA nephropathy.
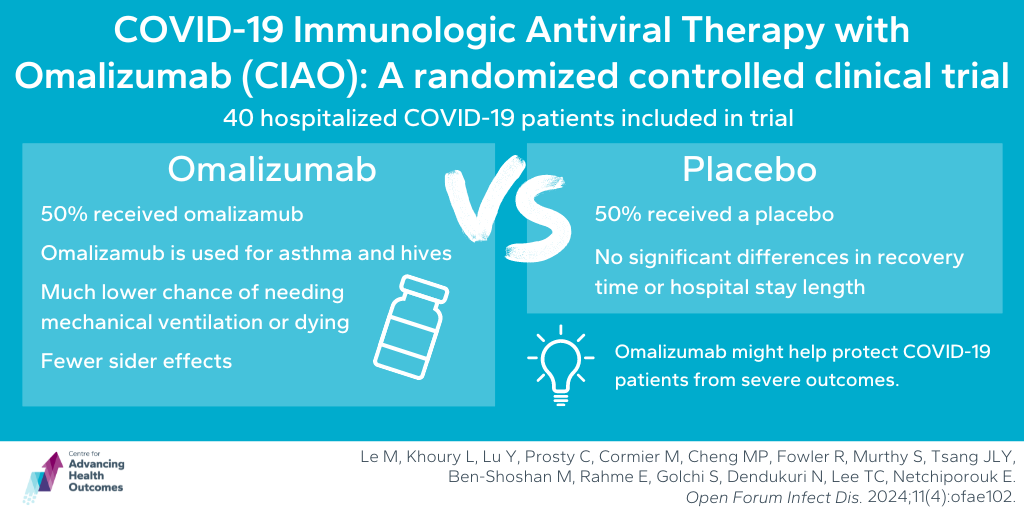
Asthma drug omalizumab could help patients with COVID-19
Le M, Khoury L, Lu Y, Prosty C, Cormier M, Cheng MP, Fowler R, Murthy S, Tsang JLY, Ben-Shoshan M, Rahme E, Golchi S, Dendukuri N, Lee TC, Netchiporouk E. COVID-19 Immunologic Antiviral Therapy With Omalizumab (CIAO)—a Randomized Controlled Clinical Trial. Open Forum Infect Dis. 2024 Feb 23;11(4):ofae102.
Advancing Health’s Dr. Srinivas Murthy worked on a clinical trial which investigated omalizumab, a monoclonal antibody medication typically used for conditions like chronic hives and asthma, to see if it could help patients hospitalized with COVID-19 pneumonia. The team conducted a trial with 40 hospitalized patients, with half receiving omalizumab and half receiving a placebo, alongside standard care. The main goal was to see if omalizumab could reduce death or the need for mechanical ventilation by day 14. Their results showed that those who received omalizumab had a 90 per cent lower chance of needing mechanical ventilation or dying compared to the placebo group. Additionally, by day 28, the omalizumab group had lower overall mortality. Although there were no significant differences in recovery time or hospital stay length, fewer side effects were reported in the omalizumab group and there were no serious issues related to the medication. This suggests that omalizumab could help protect COVID-19 patients from severe outcomes.
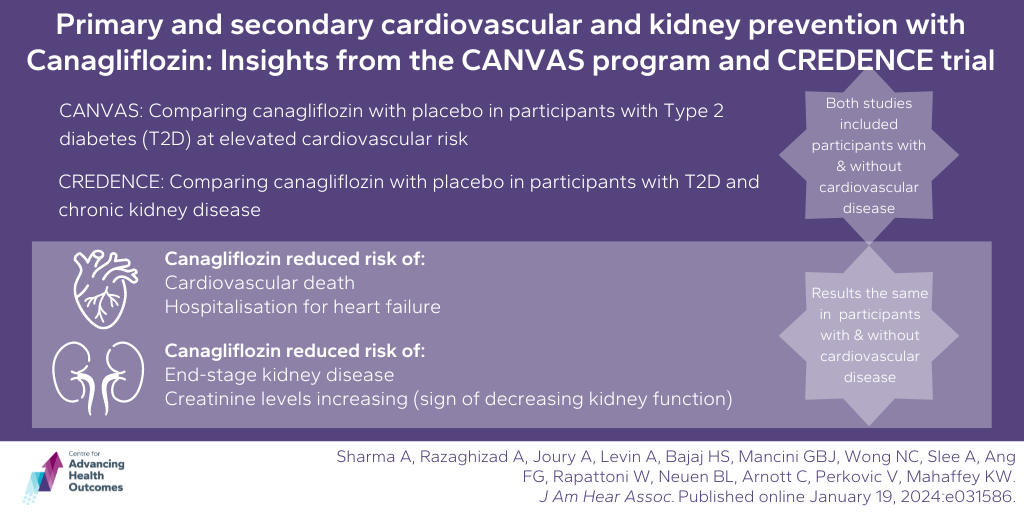
Canagliflozin could be used to prevent heart and kidney complications in people with type 2 diabetes
Sharma A, Razaghizad A, Joury A, Levin A, Bajaj HS, Mancini GBJ, Wong NC, Slee A, Ang FG, Rapattoni W, Neuen BL, Arnott C, Perkovic V, Mahaffey KW. Primary and Secondary Cardiovascular and Kidney Prevention With Canagliflozin: Insights From the CANVAS Program and CREDENCE Trial. J Am Hear Assoc. Published online January 19, 2024:e031586.
This research team, including Advancing Health Scientist Dr. Adeera Levin, studied data from two large clinical trials to see how a medication called canagliflozin affects people with type 2 diabetes. The two trials included thousands of patients with type 2 diabetes, both those with existing heart problems and those without. The team found that canagliflozin helped lower the risk of heart issues and kidney disease in both groups of patients, whether they had previous heart problems or not. The medication worked similarly well for both groups, which suggests it could be useful for preventing heart and kidney issues in people with type 2 diabetes, regardless of their medical history. This study highlights the impact of canagliflozin in treating and preventing complications in people with type 2 diabetes, however, more research is needed to determine the best way to use it in different patient populations.