The Evidence Speaks Series is a recurring feature highlighting the latest in Advancing Health research. This series features summaries of select publications and is designed to keep media and the research community up to date with the Centre’s current research results in the health outcomes field.
To ensure this research is quick and easy to share, you are welcome to save the social cards and use as you see fit.
Genetic testing may offer improved outcomes and substantial cost savings in the treatment of major depression
Ghanbarian S, Wong GWK, Bunka M, Edwards L, Cressman S, Conte T, Price M, Schuetz C, Riches L, Landry G, Erickson D, McGrail K, Peterson S, Vijh R, Hoens AM, Austin J, Bryan S. Cost-effectiveness of pharmacogenomic-guided treatment for major depression. Can Med Assoc J. 2023;195(44):E1499-E1508.
Advancing Health Research Associate, Alison Hoens, joined Dr. Shahzad Ghanbarian and team to investigate the cost-effectiveness of using pharmacogenomic testing to guide the prescription of antidepressants for patients with major depressive disorder in B.C. Pharmacogenomic testing can help us understand how a person’s genetics may influence their response to drugs. The team used a simulation model considering patient characteristics (e.g., the variation in genes that influence the metabolism of antidepressant medications) and data from systematic reviews and expert opinions to compare the projected journey of 194,149 adults with and without pharmacogenomic testing over a 20-year period. The findings indicated that implementing pharmacogenomic testing for adult patients with moderate to severe major depressive disorder could save the provincial health system $956 million ($4,926 per patient) and result in health improvements of 0.064 life-years and 0.381 quality-adjusted life-years per patient. These benefits would primarily stem from preventing or delaying the development of treatment-resistant depression. The team estimated that 37 per cent fewer patients would develop treatment-resistant depression over 20 years if pharmacogenomic-guided care was used, and patients would spend 15 per cent more time without depressive symptoms, resulting in fewer deaths and hospital admissions. According to the simulation, the costs of pharmacogenomic testing would be offset within about 2 years of implementation.

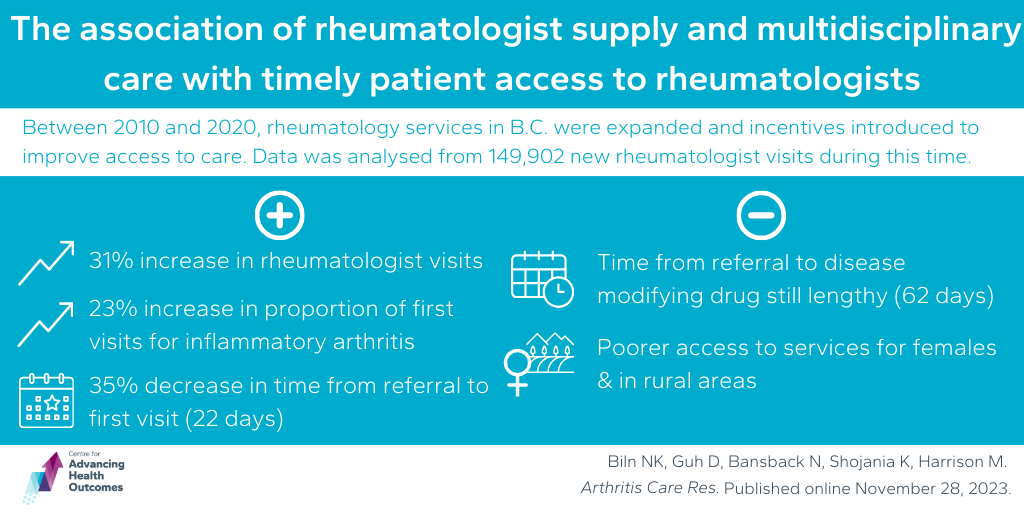
Changes in rheumatology services appear to have been impactful in improving access to services for most people in B.C.
Biln NK, Guh D, Bansback N, Shojania K, Harrison M. The association of rheumatologist supply and multidisciplinary care with timely patient access to rheumatologists: Evidence from British Columbia, Canada. Arthritis Care Res. Published online November 28, 2023.
To improve access to care in B.C., rheumatology services were expanded over the last decade, and incentives to facilitate multidisciplinary care were introduced. A team, including Advancing Health’s Drs. Daphne Guh, Nick Bansback, Kam Shojania, and Mark Harrison, investigated how this expansion of services has impacted access to rheumatology services in B.C. from 2010 to 2019. Analyzing data from 149,902 new rheumatologist visits, the study found a 31 per cent increase in visits by 2019, with the proportion of first visits for inflammatory arthritis patients rising from 28 to 51 per cent. The median time from referral to the first rheumatologist visit decreased by 22 days (35%), suggesting improved access. However, for individuals with rheumatoid arthritis (RA), the time from referral to disease-modifying drug (DMARD) treatment remained lengthy (62 days), revealing potential inequalities. The team found that females and those living outside major urban areas have poorer access to services. Overall, the study suggests improved rheumatology care access, though challenges persist, especially for certain demographics.
Navigating informed consent and data collection in Cluster Randomized Trials
Ho A, Joolaee S, McDonald M, Grant D, White MM, Longstaff H, Palsson E. Navigating Informed Consent Requirements and Expectations in Cluster Randomized Trials: Research Ethics Board Members’ and Researchers’ Views. Ethics Hum Res. 2023;45(6):31-45. doi:10.1002/eahr.500189
This study focuses on the importance of informed consent and data collection in human research, particularly in cluster randomized trials (CRTs). CRTs are often used to evaluate complex health service interventions whereby different groups of people, and their data, are involved in the trial in different ways and levels. Because of the way data is grouped, analyzed, and consented, CRTs have unique ethical considerations compared to traditional randomized control trials where individual consent is obtained. Advancing Health Scientist, Dr. Anita Ho, explores issues such as determining who is classed as active participants of CRTs, the types of consent needed, and managing data from those who haven’t given consent. The goal is to balance trust in research, respect for individuals, data integrity, and the pursuit of public good. The team conducted virtual interviews of ethics board members and researchers to shed light on how researchers and research ethics boards grapple with the ethical dimensions of data collection and use in CRTs within the evolving research landscape. Respondents argued that relying on individual-level informed consent for CRTs was inadequate and that there is a need for alternative approaches like community engagement. They also called for more education on ethical considerations, and transparent communication to ensure that participants and the public understand the importance of CRTs in advancing health services research, expressing concerns that strict consent requirements may make obtaining consent impractical, potentially compromising research benefits and perpetuating health inequities.